Feed ingredient holding explored as ASF fears continue

The Swine Health Information Center (SHIC) released the newly recalculated holding times for feed ingredients last week.
Work on the project was done in conjunction with the Pork Checkoff, National Pork Producers Council, American Association of Swine Veterinarians and the American Feed Industry Association’s (AFIA) charity, the Institute for Feed Education and Research, helped fund the project.
The work follows initial research looking at the potential for feed to transmit certain viruses including the Porcine Epidemic Diarrhea virus (PEDV) and African Swine Fever (ASF), which continues to be a concern for US swine producers.
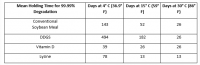
The new information calculates potential minimum holding times for ingredients including soybean meal, dried distillers grains with solubles (DDGS), vitamin D and lysine were based on new research, said Paul Sundberg, Swine Health Information Center executive director.
Information about holding as a possible way to allow 99.99% of a virus potentially present in a feed ingredient was released in October, but those times had been based on estimated half-lives of several viruses established in research that tracked the potential for viruses to be transmitted through feed or feed ingredients, he added.
“The good news is in October of 2018 that was a probably an error on the side of caution,” he told FeedNavigator.
The new holding times established in the updated research are shorter than those previously announced.
However, the information provided is the result of ongoing research, and it is not intended to be a guarantee, guideline or a recommendation, he said. “There isn’t one thing that producers or feed manufacturers can do to sterilize their feed components if they’re contaminated with viruses including African swine fever (ASF) – the issue here is similar to biosecurity on the farm,” he added.
“In biosecurity on the farm we put in a number of steps to keep bad things from getting to the pigs,” Sundberg said. “There isn’t one thing that producers can do – there isn’t something that’s 100% effective – you put a series of hurdles in place so as they’re added together it continues to lower the probability.”
Holding patterns and feed
The new information builds off work done in 2018, said Sundberg. The research re-evaluated the use of holding to maintain feed ingredients for the length of time needed for any potential virus in the ingredient to die or degrade.
Feed ingredients can be generated and handled in many ways, SHIC reported. Holding time could be an option when the product is produced in unknown conditions or if it is not sealed after it is processed.
The research is intended to address the foreign animal disease covered in previous research including foot and mouth disease, ASF, Seneca Valley A, classical swine fever and PEDv, Sundberg said.
The process is based on an understanding of the half-life of a virus, he said. The half-life is the amount of time needed for half the amount of a virus present to degrade.
The first data released regarding the potential time an ingredient needed to be kept before the potential virus would degrade was based on information gathered in a research study that did not specifically examine the half life of a virus, but provided usable information, he said.
“South Dakota State with SHCI and IFEEDER did a specific half-life study of Seneca Valley at three different temperatures,” he said. “We used Seneca Valley because it had the longest half-life, including ASF.”
The project provided a “lot more data and lot more accuracy in the calculation,” Sundberg said. The project also examined the time needed for the virus to degrade in feed ingredients during cold, moderate or warm temperatures.
Keeping the contaminated feed ingredients in a colder temperature means the virus tends to live longer, he added.
Currently, researchers at Kansas State University are doing a similar project tracking the half-life disappearance of the African Swine Fever virus specifically, he said. “They’re doing it directly with ASF to make sure – we don’t want any surprises,” he added.
The goal at this stage is to have preliminary information from that project by the end of the year, he said.
However, there are some questions remaining about the practice, and AFIA was cautious about the discussion of holding when it was first introduced. Organization members continue to maintain that more research is needed.
Talking about imported ingredients
In addition to the information about virus degradation in feed ingredients, a discussion tree regarding risk from some feed ingredients also is being shared, Sundberg said.
There are several steps that could be used as a way to minimize risk, he said. This could include steps like holding certain ingredients for a period of time, using a process control like the FDA’s Foreign Supplier Verification Program or blockchain to track the origin of ingredients and adding mitigants. However, there are no mitigants currently approved for virus reduction.
Additionally, sourcing feed or feed ingredients, when possible, from countries that do not have a disease presence for specific foreign animal diseases would could reduce the risk of contaminated feed, he said.