Promotional Features
Harnessing epigenetics to improve chick quality by maternal feeding of chelated trace mineral zinc
By Drs. Mercedes Vazquez-Anon and Juxing Chen
Novus International, Inc.
The poultry industry is always seeking ways to improve efficiency and cost of production. One way to improve efficiency is to optimize the nutrition and management of the breeder hen to affect its reproduction and the performance of its progeny. More attention has been given to broiler breeder nutrition and research and how it can improve productivity in terms of saleable chicks produced, egg fertility and hatchability than on the effect of parental nutrition on the subsequent broiler performance. For example, supplementation of chelated trace minerals such as zinc, copper and manganese in broiler breeder diets has been shown to improve pullet uniformity and livability, egg production, eggshell quality, mineral deposition in egg yolk, hatchability and chick livability, as well as reduce embryonic mortality, however, the molecular mechanism by which maternal nutrition impacts progeny health remains to be determined in detail.
Recently, new research has provided evidence on how some micro nutrients in the breeder hen diet can improve the livability and immune system of the progeny (Bergohff et al., 2013), providing new opportunities to help the chick develop the immune system in the face of the antibiotic-free environment. For example, maternal supplementation of 25-hydroxy vitamin D3 is shown to increase hatchability and chick innate immunity towards E. coli. challenge. Supplementation of zinc is shown to improve humoral and cellular immunity and aid the progeny’s immune response to pathogenic challenges (Kidd, 1992 and 1993). The combination of zinc and manganese is shown to affect the livability of the progeny (Virden et al., 2003). Of the trace minerals, zinc, especially in its chelated form, fed to the breeder hen can improve the livability of the young chick by modulating the cellular and humoral immune response and reducing intestinal inflammation(Li et al., 2015).
Maternal zinc supplementation modulates offspring adaptive immune system
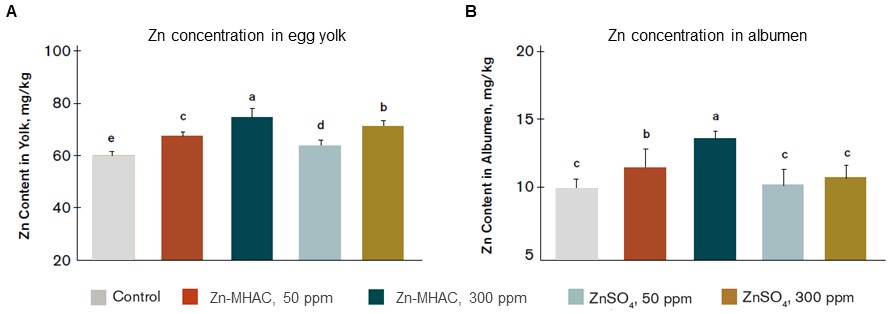
In chickens the adaptive immune system is functionally immature and development of offspring immunity can be achieved by exposing the breeder to diseases through vaccines to enhance transmission of immunity via antibodies in the egg yolk. Maternal zinc is stored in the egg yolk, yolk sac and albumen prior to its delivery to the embryo’s liver from where it is distributed to the developing organs as needed. In Li et al.’s work (2015), broiler breeder hens (45 weeks old) were fed a zinc-deficient diet (0 ppm supplemental zinc) for 2 weeks to exhaust maternal zinc and then fed commercial (50 ppm) and high (300 ppm) levels of two sources of zinc: chelated (zinc metal methionine hydroxy chelate, Zn-MHAC) and inorganic (ZnSO4); or 0 ppm supplemental zinc for 6 weeks (Control). The progeny chicks from those hens were fed either low (20 ppm) or normal (70 ppm) levels of inorganic zinc for 6 weeks. Increasing levels of zinc in the breeder diet and in the form of chelates improved storage of zinc in the egg yolk (Figure 1A) and albumen (Figure 1B), therefore more zinc was available for embryo development, and systemic immunity in progeny birds was enhanced with higher concentration of IgY in offspring chicks at 1 day of age, and higher antibody titer of Newcastle disease virus and infectious bursal disease virus in offspring chicks at 28 days of age (data not shown).
Figure 1. Zinc concentration in egg yolk and albumen in breeder hens fed the same basal diets supplemented with 0, 50, 300 ppm ZnSO4 or 50, 300 ppm Zn-MHAC. Treatments with different letters were significantly different (P < 0.05).
Maternal zinc supplementation modulates offspring local immune system in intestine
The intestine is the largest immune system of the body and plays a major role in fighting infection; however, the functional development of gut-associated lymphoid tissues in neonatal chickens is slow with the functional maturation occurring in the second week of life (Bar-Shira and Friedman, 2006). Recent work has been focusing on the mechanism by which maternal zinc supplementation modulates the immune system of the offspring.
With recent advances in epigenetics, we better understand the underlying molecular mechanism by which maternal zinc can modulate gene expression of chicks and improve immune development. Epigenetics represents changes in the gene expression independent of the DNA sequence. DNA methylation and histone acetylation are the two most common types of epigenetic modification, which in general repress and activate gene expression, respectively. These changes occur as a result of beneficial characteristics being passed from the parent to the offspring in utero, allowing the progeny to meet the demands of its environment throughout its life.
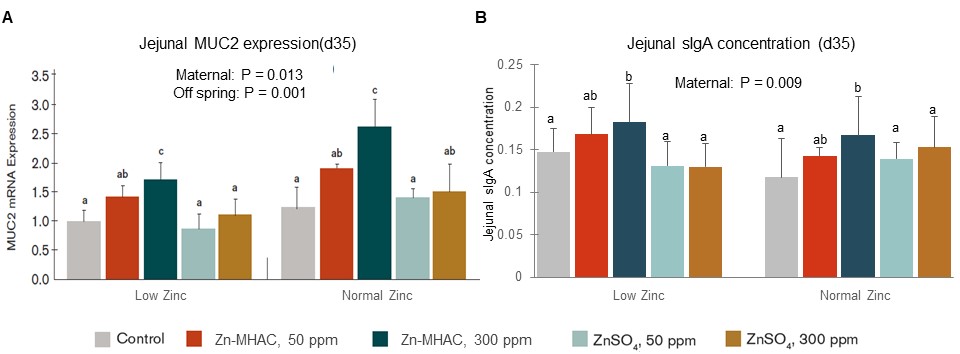
In Li et al.’s work (2015), compared to zinc-deficient or inorganic zinc treatments, chelated zinc supplementation in breeder hens was shown to be better at improving gut barrier function by increasing MUC2 gene expression (Figure 2A) and enhancing mucosal immunity by increasing secretory IgA production in progeny chicks at 35 days of age fed either low or normal zinc diet (Figure 2B). These results suggest zinc supplementation in the breeder diet improves gut barrier function and mucosal immunity of progeny chicks and zinc in the chelated form is more effective than in the inorganic form.
Figure 2. Zinc supplementation in broiler breeder hens increased mucosal MUC2 gene expression (A) and secretary IgA (sIgA) concentration (B) in jejunum of offspring chicks at 35 days of age hatched from breeder hens supplemented with 0, 50, 300 ppm ZnSO4 or 50, 300 ppm Zn-MHAC and fed diets with either normal low zinc or normal zinc. Treatments with different letters were significantly different (P < 0.05).
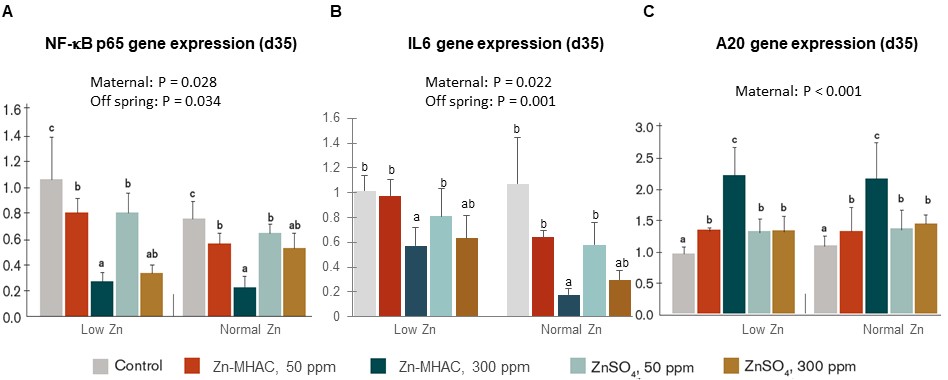
Research in humans suggests zinc suppresses inflammation by inducing expression of A20, which down-regulates the nuclear factor-κB (NF-κB) signaling pathway (Prasad et al., 2011). NF-κB signaling pathway is a key pathway that regulates inflammation induced by external stimuli like bacteria and infection. Zinc finger protein A20 can negatively regulate the inflammatory response by degrading factors of NF-κB signaling cascades (Catrysse et al., 2014). To see if the same could happen in poultry, the expression of A20, NF-κB and IL6 genes were measured in jejunum of progeny birds in Li et al’s study. Chelated zinc supplementation in breeder hens exhibited greater effect than zinc sulfate in reducing gene expression of NF-κB subunit p65 (Figure 3A) and its downstream inflammatory cytokine IL6 (Figure 3B), as well as increasing A20 gene expression (Figure 3C) in progeny jejunum. The findings suggest that zinc supplementation in breeder diets reduces gut inflammation in progeny birds by up-regulating expression of A20, a negative regulator of NF-κB signaling pathway.
Figure 3. Zinc supplementation in broiler breeder hens increased A20 gene expression and reduced NF-κB and IL6 gene expression in jejunum of offspring birds at 35 days of age hatched from breeder hens supplemented with 0, 50, 300 ppm ZnSO4 or 50, 300 ppm Zn-MHAC and fed diets with either normal low Zinc or normal Zinc. Treatments with different letters were significantly different (P < 0.05).
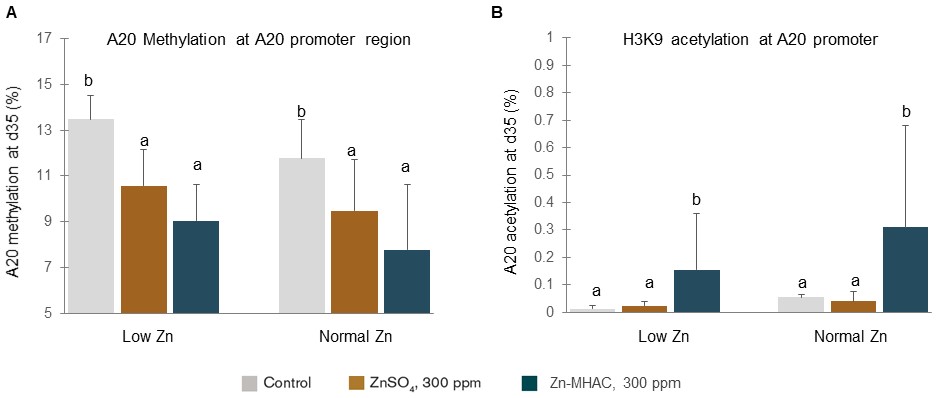
To determine the molecular mechanism by which zinc regulates A20 gene expression, researchers measured DNA methylation and histone H3K9 acetylation at A20 promoter region in progeny jejunum. Both chelated zinc and zinc sulfate significantly reduced DNA methylation at A20 promoter (Figure 4A). Chelated zinc significantly increased histone H3K9 acetylation at A20 promoter (Figure 4B). Because methylation represses gene expression and histone H3K9 acetylation activates gene expression, reduction of methylation and increase of histone H3K9 acetylation at A20 promoter region by zinc supplementation would up-regulate A20 gene expression, which explains the mechanism by which zinc supplementation increased A20 mRNA levels shown in Figure 3C.
Figure 4. Zinc supplementation in broiler breeder hens reduced DNA methylation and increased histone H3K9 acetylation at A20 promoter region in jejunum of offspring birds at 35 days of age hatched from breeder hens supplemented with 0, 300 ppm ZnSO4 or 300 ppm Zn-MHAC and fed diets with either normal low zinc or normal zinc. Treatments with different letters were significantly different (P < 0.05).
Research shows that breeder nutrition, management and environment have an impact in the development of progeny. Breeder hen nutrition is a way to prepare the chick’s immune system post-hatching, especially in an antibiotic-free environment. Zinc supplementation in breeder hen diets not only improves breeder reproduction as shown by better egg production and egg quality, higher hatchability and lower embryonic mortality, but also enhances progeny chick quality, immune development and growth.
Recent work by Li et al. delineated the mechanism by which zinc supplementation in breeder hens improves immunity of progeny chicks. Maternal zinc supplementation improved gut barrier function, mucosal and systemic immunity, and reduced gut inflammation of progeny chicks by epigenetic modification on the promoter of A20, an anti-inflammatory protein. The higher levels of zinc tested together with a chelated zinc source appeared to offer the most consistent effect in epigenetic modulation of A20 promoter. Feeding zinc, especially in the chelated form, to broiler breeders improves the chance of better livability in chicks due to a more developed immune system and provides a tool to better prepare the chick for optimal performance in an antibiotic-free environment.