MEPs oppose GM herbicide resistant soybean imports

Dow AgroSciences is seeking approval for the import, processing, and food and feed uses of soybean DAS-68416-4 but not trait cultivation within the EU.
The EU risk assessor, the European Food Safety Authority (EFSA), adopted a favorable opinion on the safety of DAS-68416-4 in January this year.
However, the Environment, Public Health and Food Safety (ENVI) committee yesterday (11 July) adopted a non-binding resolution against authorization of the GM soybean trait. It was backed by 40 votes to 24 with one abstention.
Moreover, the vote of the Standing Committee on the Food Chain and Animal Health on 12 June delivered no opinion on whether to approve the GM trait.
Concerns over herbicide related risks
The ENVI committee members cited concerns raised by independent studies in relation to the risks of the active ingredient of 2,4-Dichlorophenol herbicide, to which DAS-68416-4 is resistant, as regards embryo development, birth defects and endocrine disruption.
They also argued the other herbicide to which the GM soybean is resistant, Glufosinate ammonium, is classified in the EU as toxic for reproduction.
EU approval of the import of the GM trait into the bloc would lead to a hike in its cultivation in third countries, and to an increase of the use of 2,4D and glufosinate herbicides, they added.
EFSA opinion
EU countries are able to give input to EFSA on its GMO assessments during a commenting period. The MEPs said the feedback given on DAS-68416-4 showed serious concerns about the application and the risk assessment data, which “do not provide sufficient information to exclude adverse effects."
Testbiotech, the Germany based not for profit group set up to assess the impact of biotechnology in 2008, criticised the EFSA opinion on the soybean trait, claiming it was “not acceptable in its present form. It does not identify knowledge gaps and uncertainties and fails to assess toxicity, impact on immune system and the reproductive system.”
EFSA concluded there were no safety, allergenicity or toxicity related risks in relation to the trait: “The compositional analysis identified no differences between soybean DAS-68416-4 and its conventional counterpart that required further assessment for food/feed safety, except for a higher lectin activity (increased up to 36%) in soybean DAS-68416-4.
“The increase in lectin activity is unlikely to raise additional concerns for food/feed safety and nutrition for soybean DAS-68416-4 as compared to its conventional counterpart and the non-GM commercial varieties.
"No concerns were identified regarding the potential toxicity or allergenicity of the newly expressed AAD-12 and PAT proteins, and no evidence was found that the genetic modification might significantly change the overall allergenicity of soybean DAS-68416-4.
“The GMO Panel concludes that soybean DAS-68416-4, assessed in this application, is as safe and as nutritious as its conventional counterpart and the non-GM soybean reference varieties tested.”
Next steps
The ENVI committee non-binding resolution will now go to a vote by the full house during the September plenary session in Strasbourg.
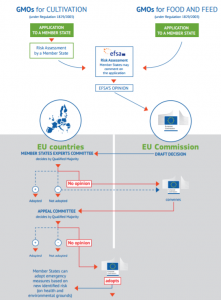
All GMOs need an authorisation before they can be placed on the EU market. This authorisation is given in the form of an implementing act under the 'examination' procedure, based on a risk assessment conducted by EFSA.
The implementing decision has to be approved by a qualified majority in a standing committee of member states' representatives. However, a qualified majority among the EU-28 has never been found, either in favor of or against a proposal for decision proposed by the Commission.
According to the applicable legislation and case law, the Commission is ultimately obliged to adopt a decision.
In this context, MEPs yesterday also called on the Commission to table a new proposal on the GM authorization process within the EU as opposed to the one it put forward in April 2015 that would have enabled individual states to restrict or prohibit the use of GM food and feed on their territory.
That draft law was opposed by Parliament in October 2015. The MEPs, along with food and feed industry stakeholders and NGOs, have long voiced concerns this law might prove unworkable or that it could lead to the reintroduction of border checks between pro- and anti-GMO countries.